Long Island's Premier Vein Treatment Center
Exceptional Care for Capillary Veins & Varicose Veins
View our 5 Star Reviews On Google!

Dr. Ninia
MD, RVT, FACS
Our Services
Our Specialized Services
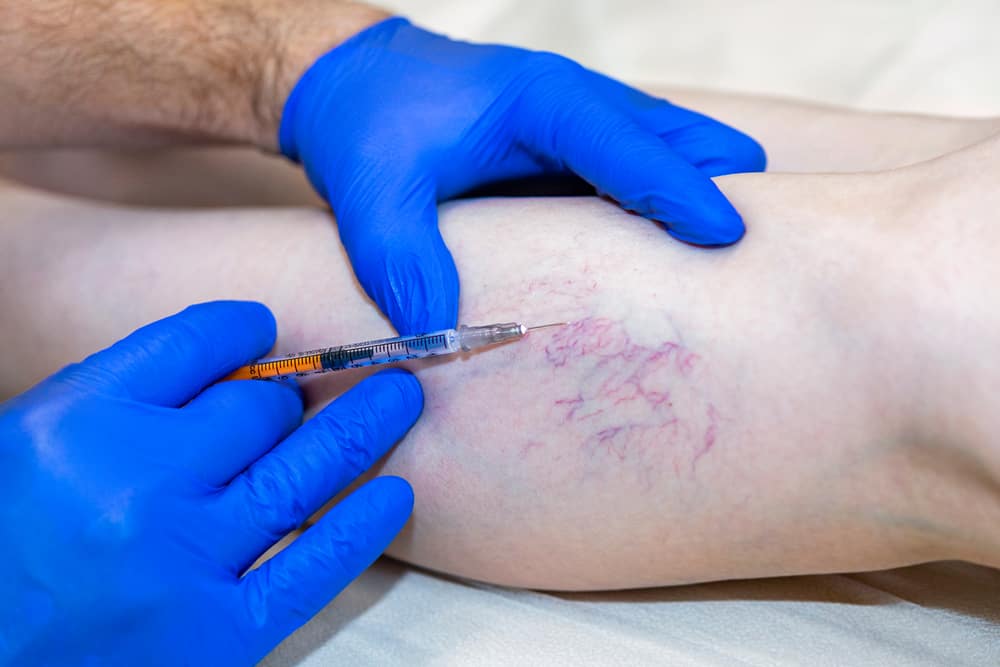
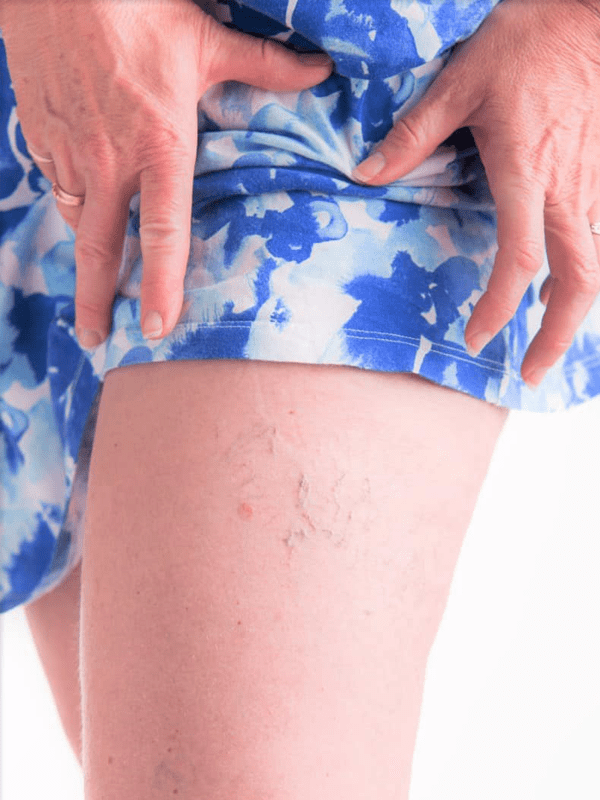
Many suffer from the discomfort of varicose and capillary spider veins. At the Varicose Vein Center in Port Jefferson Village, we specialize in their evaluation and treatment using safe, painless duplex ultrasounds to visualize and address the underlying issues, preventing further health complications.
Varicose Vein Removal
Regain confidence with our expert vein removal treatments.
Sclerotherapy
Minimally invasive procedure to treat capillary spider veins.
Laser Ablation
Cutting-edge laser treatments to address larger varicose veins.

VenaSeal
A revolutionary adhesive solution for vein closure without surgery.

Varithena
Advanced foam sclerotherapy ensuring optimal results.
Diagnostic Ultrasound
Safe and painless diagnostic tool to visualize and assess vein health.
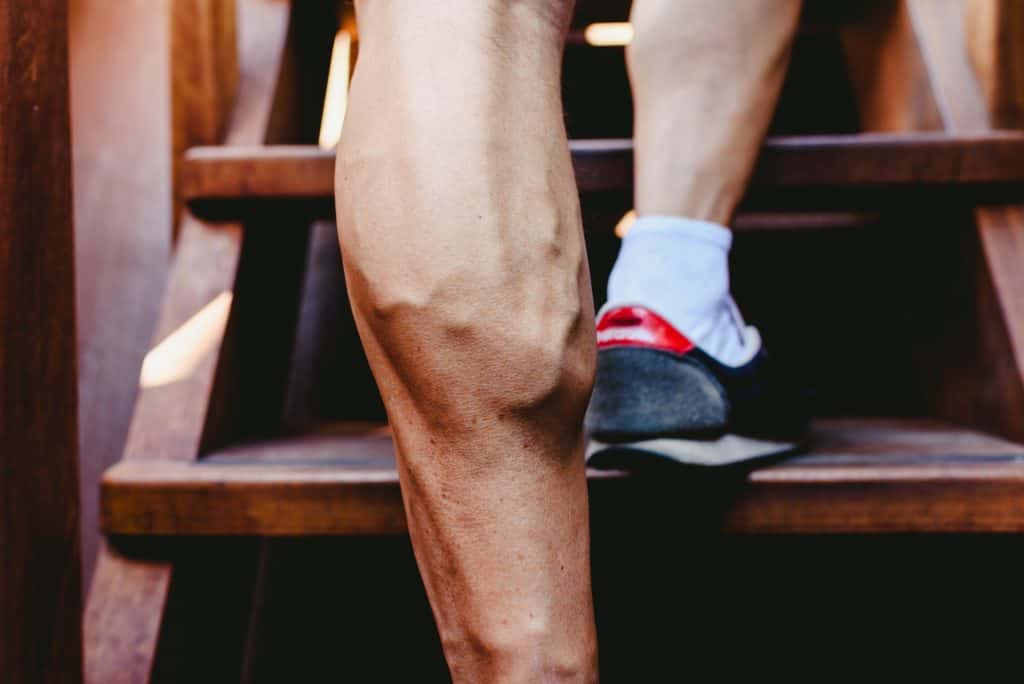
Why Varicose Veins Center
With over three decades of expertise, our center in Port Jefferson has been the beacon of hope for those suffering from vein issues. Our unwavering commitment to patient care and innovative treatments set us apart.
- 30+ Years of Trusted Experience.
- Individualized Treatment Plans
- Post-Treatment Support
- Positive Patient Outcomes
Meet Dr. Ninia
Jerry G. Ninia, MD, RVT, FACS
Dr. Ninia has been practicing Phlebology (Vascular Medicine) and OB/GYN in Suffolk County for 30 years. He grew up on Long Island and attended Adelphi University. He graduated from Wayne State University School of Medicine in Detroit, Michigan, and returning to Long Island, he completed an internship and residency in OB/GYN at Nassau University Medical Center. Afterward, he pursued additional preceptorship training in Phlebology including certification as a registered vascular technologist (RVT) with expertise in diagnostic ultrasound. He is a diplomat of the american veinist and lymphatic society.

More than just a treatment center, we are your partners in health.
Our dedicated team at Varicose Veins Center in Port Jefferson, NY, offers personalized treatment plans alongside extensive patient education and post-care guidance. If you’re battling varicose veins, reach out for expert care and solutions to achieve healthier, beautiful legs.